On June 1, the Indian Government approved US-drug major Gilead Sciences Inc's antiviral drug Remdesivir for emergency use, only up to five doses in treating moderately-ill COVID-19 patients. The drug Remdesivir is to be administered intravenously. It is the first drug to show improvement in formal clinical trials.
Gilead grants royalty-free licenses to Indian pharma companies for the production of Covid-19 drug Remdesivir until the World Health Organization (WHO) declares an end of Covid-19 pandemic, or until an alternative product or vaccine is approved and available to treat the virus, whichever is earlier.

The licensees are allowed to set their own prices for the generic products they produce. A new drug application (NDA) has been signed by Gilead for marketing this new drug in a country. As of now, the supply of Remdesivir is limited, the company aims to scale up production in July and the supply will thereafter continue to increase towards the end of this year and into next year.
However, the timelines to get Remdesivir quickly to the Indian patients are completely dependent on the licensees, and Gilead does not control the process. The individual licensees will be required to file applications with the Central Drugs Standard Control Organisation (CDSCO) to manufacture and sell Remdesivir for Covid-19 patients in India while waiving the requirement for undertaking local clinical trials.
Considering the drug is still not "the treatment plan" for Covid-19 patients and is currently under trial, a report earlier suggested that licensees will have to sell the final product only to government institutions for now. We are not sure if this still implies, but it seems pharma distributors are making most of the opportunity by pricing the experimental drug at Rs 7000 per vial.
While this move by the US-drug major Gilead is intended in hopes that huge volumes and competition will drive costs down, what will be the effect on the ground? Will the final product be overpriced for end-consumers (COVID-19 patients)? Would the Indian government intervene to make the drug affordable, until an alternative drug or vaccine is available for the virus?
Gilead's Remdesivir authorized for emergency use only
The U.S. Food and Drug Administration (USFDA) last month granted authorization for Remdesivir for emergency use, and it has received approval from the Japanese health regulators. The drug is now being administered in some countries for compassionate use on severe Covid-19 victims.
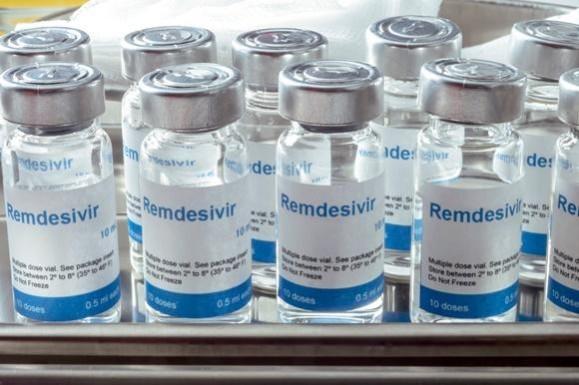
The drug is at the forefront of the battle against COVID-19, which has no approved treatment or vaccine thus far. The Drugs Controller General of India (DGCI) approval comes a day after the U.S. drugmaker reported Remdesivir, an experimental drug showed modest benefit in patients with severe COVID-19 symptoms in a five-day course. The regulator does not support extended use of the drug for a period of up to 10 days based on existing evidence presented to the drug controller at the time of approval.
Gilead Inc. entered into non-exclusive licensing pacts last month with few Indian drug majors namely Cipla, Jubilant Life Sciences, Hetero Drugs, and Mylan for production of Remdesivir and expand the supply of this experimental drug. While these companies are ready with the final product, they await approval from the Drug Controller General of India to start manufacturing and selling the product.
The cost factor: Making the drug affordable for masses
As per the cost-benefit modeling done by the Institute for Clinical and Economic Review, Gilead's own brand Veklury is priced at $4460 for a full course of treatment. But the company has not yet fixed the final price for the US market. According to a news report, the global sales of Remdesivir is likely to touch $1.9 billion by the end of 2020.
The Government of India has not yet taken a view on the final pricing of Remdesivir, but the drug pricing regulator can invoke paragraph 19 of the Drug Price Control Order (DPCO) 2013 that empowers the National Pharmaceutical Pricing Authority (NPPA) to fix the price of any medicine in the public interest or under extraordinary circumstances.
The government can increase or decrease the ceiling price or retail price of the drug, irrespective of the annual wholesale price index for that particular year. The DPCO further allows NPPA to seek additional information or make an inquiry about the efficacy of the drug to treat moderately-ill Covid-19 patients if considered necessary in the public interest.
To boost the supply of Remedisvir in India, Gilead will be working closely with health regulators to provide guidance based on local incidence and severity of the disease. The US-drug major is now working on the development of easier-to-administer versions of Remdesivir that can be used outside of hospitals, perhaps ones that can be inhaled, after clinical trials show moderate effectiveness for the drug given by infusion.
For critically-ill Covid-19 patients, Roche and Eli Lilly and Co are testing drugs in combination with Remdesivir. Meanwhile, the European and South Korean authorities are looking at imports of the drug as an immediate remedial measure, but Gilead is yet to gain regulatory approval in either of these markets.

















