
Astrophysicists have believed that ice giant planets Neptune and Uranus undergo rains of diamonds.
ALSO READ: NASA will explore icy moons Europa and Enceladus for alien life: 7 things to know
The ice giants comprise moderately minute rocky cores which are surrounded by a mantle made up of ammonia, water and methane ices. It is blanketed by an atmosphere consisting hydrogen, helium and methane gas along with an upper atmosphere and cloud tops.
This research was carried out by researchers at Helmholtz-Zentrum Dresden-Rossendorf (HZDR), who joined hands with colleagues from Germany and the US.
According to the researchers, the diamond showers take place on these ice giants because of extreme temperatures and severe pressure which reign up to 10,000 kilometres (6213.71 miles) under the surface of these planets. It breaks the hydrocarbons which lead to the formation of diamonds. These diamonds then fall as rain in the planet.
"So far, no one has been able to directly observe these sparkling showers in an experimental setting," says Dr Dominik Kraus, a scientist at Helmholtz Zentrum Dresden-Rossendorf and lead author of the study on the results.
"In our experiment, we exposed a special kind of plastic – polystyrene, which also consists of a mix of carbon and hydrogen – to conditions similar to those inside Neptune or Uranus," he added.
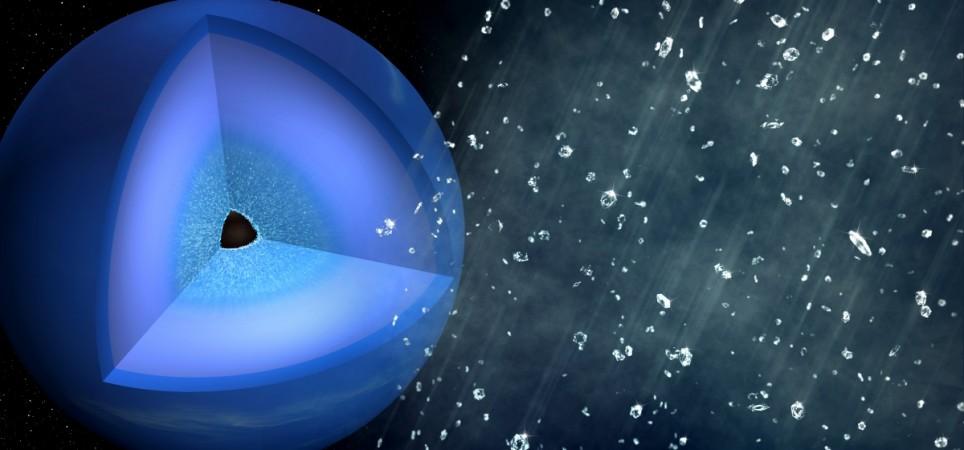
Here's all you need to know:
- The researchers recreated the process in a lab and accomplished this discovery. The astrophysicists analysed polystyrene – a plastic material – which is made using hydrogen and carbon as the main components.
- Two shock waves were created in the plastic by using high-powered optical laser using the right combination of pressure and temperature. The first shock wave was slower and smaller, whereas the second shock wave was stronger.
- "When the shock waves overlap, that's the moment the pressure peaks and when most of the diamonds form," Kraus said.
- The researchers then explored the reaction with pulses of X-rays from Linac Coherent Light Source (LCLS) that lasted for a span of only 50 femtoseconds. It just took fractions of a second for the tiny diamonds to form and the researchers used a technique called 'femtosecond X-ray diffraction' to see and analyse these diamonds.
- The scientists accumulated the data about the chemical reaction while it took place as well as the size of these diamonds with the help of X-ray snapshots.
"For this experiment, we had LCLS, the brightest X-ray source in the world," said Siegfried Glenzer, professor of photon science at SLAC and a co-author of the paper.
"You need these intense, fast pulses of X-rays to unambiguously see the structure of these diamonds because they are only formed in the laboratory for such a very short time," Glenzer added.

















