While India has witnessed no respite from the COVID-19 pandemic, a countrywide outbreak of Black fungus infections among survivors and patients has added to the coronavirus-related death toll. With the shortage of drugs making treatment of the fungal infection—which is also known as mucormycosis—very difficult, Liposomal Amphotericin B (LaMB), a drug developed by B. Srikantha Annappa Pai has emerged as a lifesaver.
The medication, whose development was spearheaded by Pai way back in 2010-11, is saving lives across the country, and the researcher says that he is "grateful" for it. So who is Srikantha Annappa Pai and how does the drug engineered by him benefit sufferers of mucormycosis?
Humble Roots

Pai was born in Gangolli, a village in Kundapura Taluk in Udupi district, Karnataka. He received a Bachelor's degree in Pharma from the Government Pharmacy College in Bengaluru. Following this, he completed his post-graduation in Manipal. With employment opportunities being slim in his home state, he moved to Mumbai seeking better options.
"Job opportunities in Karnataka weren't great. So I moved to Mumbai and stayed with my sister and started hunting for work. After several interviews, I landed a job with Bharat Serums," Pai told News18. He worked in the company for 17 years and was also the head of the research and development team at Bharat Serums and Vaccines Limited in Mumbai. In total, Pai has over 35 years of experience in the pharmaceutical industry.
Perfecting a Life-saver
LaMB, which is also known as AmBisome, is a lipid-associated formulation of amphotericin B, a broad-spectrum antifungal agent. It can act against clinically important molds and yeasts including Aspergillus, Candida and Zygomycetes (filamentous molds). LaMB was initially developed for the improvement of tolerability of the drug amphotericin B deoxycholate, which was considered the gold standard of antifungal treatment for several decades.
The patent for the drug to treat mucormycosis was owned by US-based pharmaceutical company, Gilead Sciences, which also manufactures the now COVID-19 drug—Remdesiver. However, the patent for the drug expired in 2008. "India didn't have the necessary technology to create an alternative and so lots of research and development was necessary. So it took two long years for the team to finally come up with an efficient product," explained Pai.
He added that an SoP (standard-operating procedure) for the re-development of the drug was unavailable, with only its fundamental formula made available by the company. "They will only tell if the drug is made right or wrong. Hence, we had to do every other research and reach the clinical trial phase. We also had to get permissions from the FDA and European Medical Agency before the trials. After all this, the drug was finally released in 2010-11," recounted Pai.
How does LaMB work?
Mucormycosis is caused by a group of fungi known as mucormycetes. The fungal infection usually affects people with immunosuppressive diseases such as HIV/AIDS, and other diseases such as kidney disease, diabetes, liver or cardiac disorders. Individuals on immunosuppressant medications for conditions such as rheumatoid arthritis are also at an increased risk of the infection.
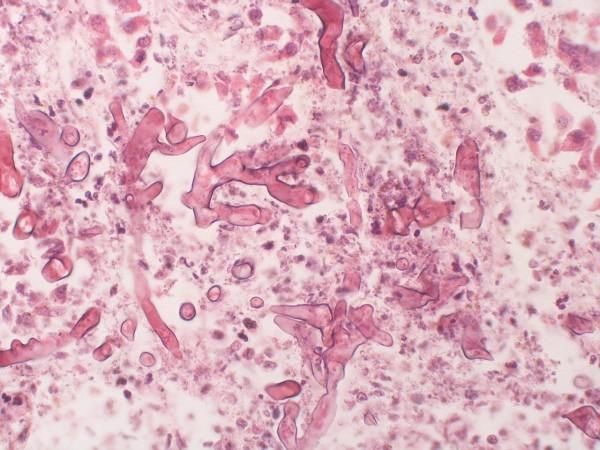
In the case of COVID-19 patients, the infection has widely been reported in patients with a history of diabetes and those who received steroids as a part of their treatment of the viral infection. "Mucormycosis majorly targets the lungs, liver, and spleen. Amphotericin B is the source of injections that are available everywhere. Four different varieties of injections have been developed from this primary source," explained Pai.
The first injection is the traditional or the original product. Liposomal amphotericin injection is the second iteration of the drug and is 75 percent more effective than the regular jab. The third form is lipid complex, which is 20 times more effective than the second variety. Finally, amphotericin emulsion—the last variant—is 150 times more efficacious than the regular injection, noted Pai.
Along with the patent for amphotericin and lipid complex, Pai holds 16 other patents. Some of these include protocol drugs that are utilized for the administration of anesthesia during surgical procedures. Retired, he currently resides in Mumbai with his family.

















