A team of researchers from the National Institute for Allergy and Infectious Diseases (NIAID) in US have announced that their experimental Ebola vaccine had succeeded in producing promising results in humans.
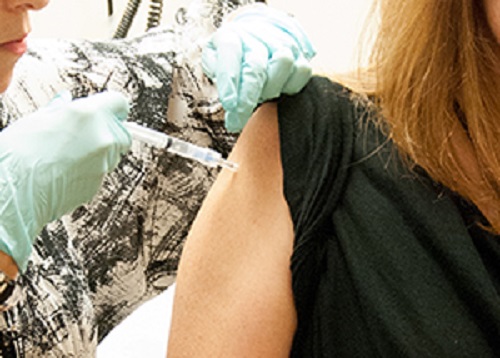
A clinical trial conducted on 20 healthy individuals, aged between 18 and 50, showed that the vaccine was safe and was also highly effective in producing the immune system response needed to fight the deadly disease.
Scientists from the NIAID Vaccine Research Center (VRC) and Okairos, a Swiss biotechnology company acquired by GlaxoSmithKline (GSK) have been credited for the ground-breaking innovation.
The vaccine works by delivering segments of Ebola virus genetic materials from the Zaire and Sudan strains, through a carrier virus -- chimpanzee-derived adenovirus 3 or cAd 3.
Researchers, while assuring the safety of the vaccine, said that the vaccine was totally free from Ebola virus and does not cause any Ebola infection.
During a clinical trial conducted at the NIH Clinical center in Maryland, ten participants received a lower dose of the vaccine, while the other half were given a higher dose of the same vaccine.
On testing the participants' blood samples, researchers found that the vaccine was highly effective in producing anti-Ebola antibodies within a short period of one month.
Trials conducted earlier by VRC scientists have shown that the vaccine, through the production of antibodies and T cells, can induce immune system response required for fighting the disease. In that September study, vaccinated monkeys did not develop the infection when exposed to high level of Ebola virus.
In the current trial, blood samples of nine participants contained CD8 T cells, a sign which indicated that the vaccine was successful in producing immune response.
"We know from previous studies in non-human primates that CD8 T cells played a crucial role in protecting animals that had been vaccinated with this NIAID/GSK vaccine and then exposed to otherwise lethal amounts of Ebola virus," Julie E. Ledgerwood, a VRC researcher who led the trial, said in a news release.
"The size and quality of the CD8 T cell response we saw in this trial are similar to that observed in non-human primates vaccinated with the candidate vaccine."
Researchers expected that the study published in the New England Journal of Medicine will help prevent similar outbreaks of Ebola in the future.
"The unprecedented scale of the current Ebola outbreak in West Africa has intensified efforts to develop safe and effective vaccines, which may play a role in bringing this epidemic to an end and undoubtedly will be critically important in preventing future large outbreaks," NIAID Director Anthony S. Fauci, explained.
Considered to be one of the largest outbreaks of Ebola in the human history, the deadly disease has already infected 15,935 people across the world and killed 5,689.