The SARS-CoV-2 virus is evolving at a pace that is becoming difficult for the scientific community to keep up with. Several variants, especially variants of concern (VOCs), have developed mutations that enable their evasion from antibodies—acquired through vaccination or infection—with ease. Providing some good news, scientists have announced the discovery of a 'super antibody' that can tackle a wide range of SARS-CoV-2 variants causing COVID-19, and other closely related coronaviruses.
According to the study by an international team of researchers, an antibody, S2H97, isolated from survivors of COVID-19 and SARS showed strong neutralizing action against the SARS-CoV-2 and other related viruses. The antibody was found to target a part of the 'spike' protein where the binding with cells takes place— RBD (receptor-binding domain). S2H97 was able to not just block the spread of the coronavirus between cells under laboratory conditions but also provide protection against it in an animal model.
"These data highlight principles underlying variation in escape, breadth, and potency among antibodies targeting the RBD, and identify epitopes and features to prioritize for therapeutic development against the current and potential future pandemics," wrote the authors. The study was published in the journal Nature.
Variants and Antibody Evasion
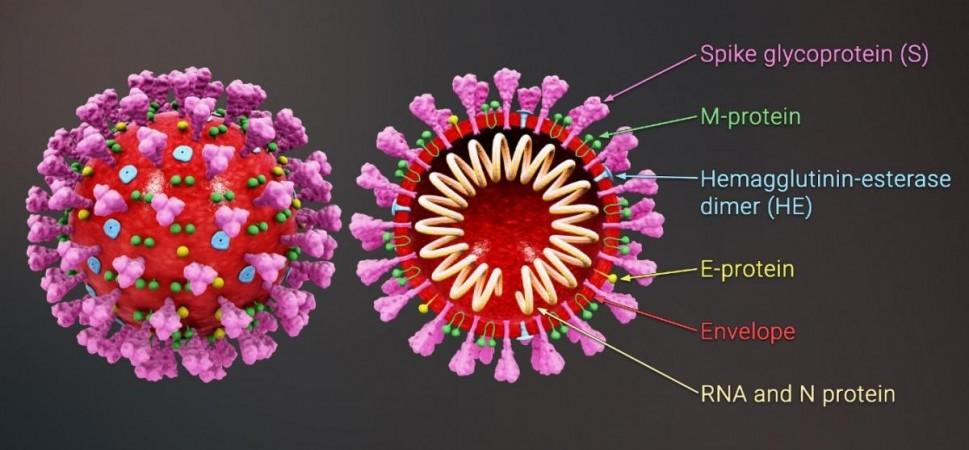
In the 20 months that the COVID-19 pandemic has ravaged the world, the SARS-CoV-2 virus has mutated thousands of times, with some resulting mutants becoming more potent than the original strain that arose in China. Most of these alterations have occurred on the 'spike' or 'S-protein'. The spike is a hook-like structure found on the surface of the SARS-CoV-2 (and also other related viruses) that the pathogen uses to invade cells. It gains entry into cells by mostly latching on to a receptor present in several cell types—ACE2 (angiotensin-converting enzyme 2).
Most of the mutations in the spike are located on its RBD. It is the region where the 'docking' between the spike and the cell occurs. The alterations in this specific site have endowed several variants of the SARS-CoV-2 with the ability to evade antibodies acquired either to infection or vaccination. This has become a cause for concern among researchers as well as governments across the world.
The SARS-CoV-2 belongs to a group of viruses known sarbecoviruses as which also includes the SARS-CoV. The group also comprises SARS-CoV-like coronaviruses that are found in numerous species of bats and other mammals such as pangolins and civets. Through the current research, the team aimed to identify antibodies that could have a fatal action against multiple species of sarbecoviruses.
Seeking a Powerful Antibody
For the study, the authors evaluated 12 antibodies that had been isolated from individuals who had contracted either SARS-CoV-2 or SARS-CoV, by one of the collaborating institutions. These antibodies attach themselves to the RBD. Several antibody treatments for COVID-19 also focus on this region of the spike. The team collated a list of mutations—numbering in thousands—that had been identified on the binding domains of several variants of SARS-CoV-2.
Additionally, they recorded mutations that have been found in the binding domains of numerous closely related sarbecoviruses. These viruses are known to mostly cause respiratory illnesses. Following this, they analyzed the manner in which the cataloged mutations affected the ability of the 12 antibodies in attaching themselves to the binding site.
Potent Neutralization of SARS-CoV-2
Among the 12 tested antibodies, one emerged as the most potent one— S2H97. It exhibited the strongest binding capacity and was able to bond with the RBD regions of all the sarbecoviruses that it was pitted against. This included several variants of the SARS-CoV-2 as well. In vitro cellular experiments revealed that S2H97 could block the spread of SARS-CoV-2, its variants, and other sarbecoviruses, among cells. Importantly, when the prophylactic efficacy of S2H97 was examined in hamsters, it was robust enough to protect the animals against COVID-19.
In order to understand the source of S2H97's potency, the scientists examined its molecular structure. They learnt that the antibody targets a region of the RBD that is concealed and was previously undetected. This crucial section becomes observable only when the RBD is about to bind with receptors.
Along with dubbing S2H97 a pan-sarbecovirus antibody, the authors believe that molecules that focus on this specific region on the RBD can be utilized to provide protection against a broad range of viruses. Interestingly, S2H97 is a non-ACE2-competitive antibody (i.e) it does not compete for ACE2 binding to act on the virus. Muck like other antibodies of its kind, its neutralizing capacity is reduced in cells that over-express ACE2.
The authors wrote: "Consistent with its ability to neutralize spike-mediated viral entry, S2H97 inhibits spike-mediated cell-cell fusion. Taken together, these experiments suggest that the S2H97 mechanism of neutralization involves receptor-independent conversion of S to the post-fusion state, thereby inhibiting ACE2-mediated cell entry."
Team of Strong Antibodies
While S2H97 displayed strong anti-coronavirus action, the other 11 isolated antibodies also exhibited good levels of neutralizing capacity against a variety of sarbecovirus. One RBM targeting antibody, S2E128, came second to S2H97's efficiency. Not only was S2E128 able to nullify the SARS-CoV-2 and its variants, but it also showed a high barrier to viral escape like S2H97.
Most RBD-targeting antibodies pick on mutations that are present in VOCs. In contrast, S2E12 targets mutant variants at sites that do not display considerable variation within SARS-CoV-2 that are in circulation. Therefore, it correspondingly blocks a wide range of SARS-CoV-2 variants. "As highlighted by S2E12 and S2H97 above, antibodies with a modest degree of sarbecovirus breadth show a greatly reduced frequency of escape mutations among circulating SARS-CoV-2 variants," the authors wrote.

















