Respiratory infections such as the flu or common cold are some of the most common infections that ail millions of people across the world. Most individuals recover from them easily. The infection caused by the Respiratory syncytial virus (RSV) is no different. However, the outcomes can be fatal in children and adults, and can result in hospitalization. Now, scientists have discovered that the change in the shape of the virus may affect the outcome of the disease.
According to a new study, when the RSV changes shape, from rods to spheres, an essential viral protein also changes shape. This affects the manner in which complement proteins—a system of proteins in the plasma that are triggered by pathogens directly or by pathogen-bound antibodies indirectly—undergo activation.
"These results identify antigenic and biophysical characteristics of virus particles that contribute to the formation of viral immune complexes, and suggest models for how these factors may shape disease severity and adaptive immune responses to RSV," wrote the authors. The findings were published in the journal eLife.
Leading Cause of Hospitalization
![Scanning electron micrograph of human respiratory syncytial virus (RSV) virions (colorized blue) and labeled with anti-RSV F protein/gold antibodies (colorized yellow) shedding from the surface of human lung epithelial A549 cells [Representational Picture] RSV](https://data1.ibtimes.co.in/en/full/766337/rsv.jpg?h=450&l=50&t=40)
Respiratory syncytial virus (RSV) is an infectious negative-strand RNA virus that belongs to a family of viruses known as Pneumoviridae. It causes infections of the respiratory tract in humans. The mode of transmission of the virus is the same as most respiratory viruses: through contact with droplets from infected individuals.
RSV infection is fairly common with the likeliness of contracting it multiple times. While most individuals recover from it in a few weeks, it can have fatal outcomes in infants and children (infected for the first time), and older adults (in whom immunity to the pathogen has waned). According to the WHO, RSV is one of the leading causes of hospitalizations due to acute lower respiratory infections, particularly among infants and children, across the world.
Key Target Protein
The genome of RSV encodes three membrane glycoproteins— the fusion protein (F), the attachment glycoprotein (G), and the short hydrophobic protein (SH). These proteins express themselves on the surface of cells that have been infected and are packaged to differing degrees into shed virus particles. The adaptive immune response primarily targets the F and G proteins.

In order to infect a cell, the membranes surrounding individual RSV viruses and cells must merge together through the moderation of the F protein. The infection of cells can be prevented by antibodies that bind with F protein. However, Dr. Michael D. Vahey, corresponding author of the study, highlighted that the protein is known for its shapeshifting abilities, thereby, making it extremely unstable.
Most antibodies directed against the prefusion conformation of F (pre-F) are usually capable of preventing viral entry. However, antibodies that bind to the postfusion conformation of F (post-F), often cannot block the entry of the virus.
Changing Shape of RSV
For the study, the authors developed a fluorescent system (or tags) that targeted the surface glycoproteins F and G of the RSV virus. This system enabled them to examine the interactions among the viruses and complement proteins, the proteins within the immune system that help in fighting infections.
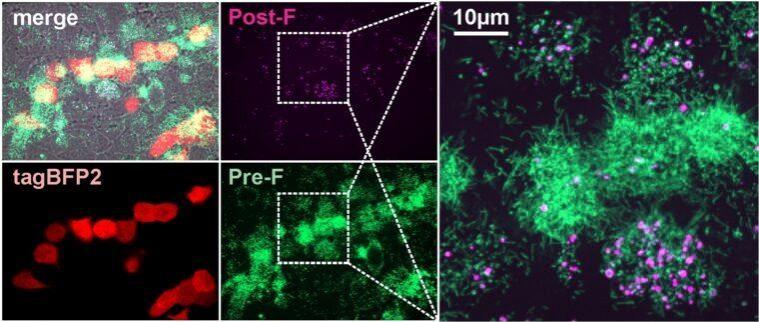
Utilizing the fluorescence-based method, they discovered that during an RSV infection, the viruses produced change shape. It was found that the pathogens transform from long, rod-shaped particles to more rounded ones. This alteration determined whether the protective complement proteins underwent activation or not.
"We found that when RSV changes shape from rods to spheres, the F protein tends to change shape, too. What we end up with is a situation where the virus particles that activate the complement system frequently have the wrong form of F on their surface. This may instruct the immune system to go after the wrong target," explained Dr. Vahey, in a statement.
According to Dr. Vahey, the findings demonstrate that the biophysical characteristics of a virus, such as its shape and size play a vital role with regards to the manner in which it is identified by the immune system. This may be a vital consideration while designing vaccines or potential treatments against viruses.








