Since the onset of the COVID-19 pandemic, scientists across the world have turned to existing drugs to combat the SARS-CoV-2 virus. One such therapeutic candidate was fenofibrate, a drug used to treat abnormal cholesterol levels. Having shown promising results against the novel coronavirus in laboratory studies, the drug was touted as a potential treatment for COVID-19. Unfortunately, scientists have now found that fenofibrate has no significant effect on the outcomes of the disease in humans.
Through a new international randomized clinical trial, researchers discovered that fenofibrate was unable to reduce the severity of disease or prevent adverse outcomes such as deaths in COVID-19 patients. The findings of the study—which was led by scientists from Penn Medicine—were published in the journal Nature Metabolism.
"Despite the promising effects of fenofibrate on SARS-CoV2, the virus that causes COVID-19, our findings convincingly showed that it is not a useful strategy for decreasing disease severity or preventing bad outcomes in patients with COVID-19," said Dr. Julio Chirinos, first author and principal investigator, in a statement.
From Fighting Fat to Novel Coronavirus
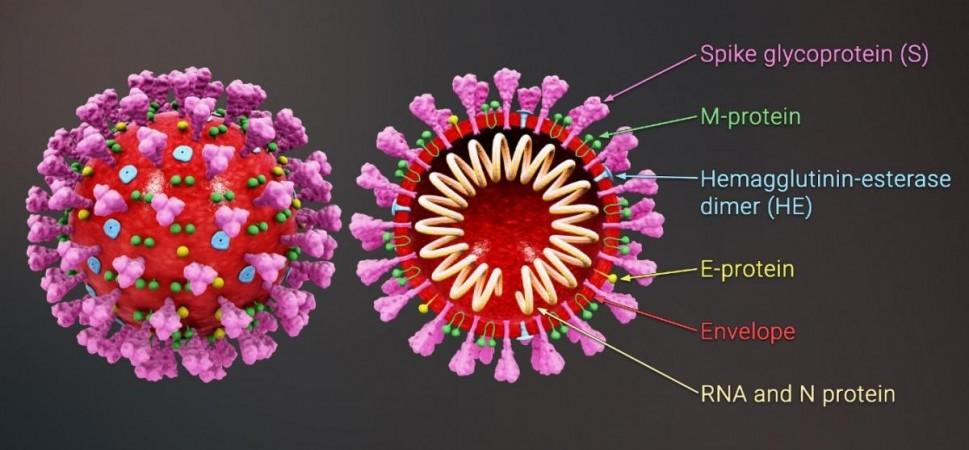
Fenofibrate belongs to a class of drugs known as fibrates. Administered orally, the compound is used to lower elevated levels of fatty substances such as cholesterol and triglycerides in the blood, and to increase the amount of "good" cholesterol or high-density lipoprotein cholesterol (HDL). It is approved by the US Food and Drug Administration (FDA), National Institute for Health and Care Excellence (NICE), and other regulatory agencies across the world.
Initial laboratory studies had suggested a link between abnormal lipid metabolism and the pathogenesis of SARS-CoV-2. The excessive cellular production of certain fat molecules such as triglycerides was found to be associated with the damage caused to cells by the novel coronavirus. When tested, fenofibrate was able to mitigate this occurrence and reduce viral replication.
Additional studies demonstrated that fenofibrate inhibited the binding between the RBD (receptor-binding domain) of the SARS-CoV-2 spike protein and the ACE2 receptor (an enzyme found on the surface of cells that is exploited by the virus to gain cellular entry). This also contributed to a decrease in viral replication. Motivated by these findings, the authors wished to investigate the effectiveness of fenofibrate against COVID-19 in humans.
Testing Efficiency In Humans
For the study, the team utilised data from 26 collaborating institutions in Europe, Western Asia, North America, and South America. They enrolled 701 participants—302 inpatients and 398 outpatients—who had experienced symptoms of COVID-19 within 14 days of contracting the infection.
They were randomly assigned to two groups: one that was treated with 145 milligrams of fenofibrate (n = 351) and the other that received a placebo (n = 350), for 10 days. The participants (male = 371, female = 330) were between the ages of 33-65. Their body mass index ranged between 22-34 kg/sq.m. Among the total participants, 102 had diabetes. 47 had heart disease, and 186 had hypertension.
A novel severity score system was used to rank the patients. Among inpatients, the system measured disease severity and factors such as the duration of hospitalisation, use of non-invasive and ventilators, and death. In the case of outpatients, additional factors such as symptom severity and time to hospitalisation were also recorded.
No Noticeable Effect

The researchers observed that in comparison to the placebo, fenofibrate had no effect on severity scores or deaths (from any cause), in addition to other metrics. While 22 deaths occurred in the placebo group,19 deaths were reported in the fenofibrate group; thereby, evidencing that the drug had no effect on severe outcomes when compared to the placebo.
Similarly, no difference was observed in outcomes up to 30 days following the initial randomization in both groups. Most of all, the findings were consistent among participants from all the countries and were not impacted by factors such as age, race, sex, body mass index, and time of initiation of treatment, among others. The team noted that there could be several potential reasons behind fenofibrate's failure in replicating its laboratory success against SARS-CoV-2 in the human study.
Dr. Jordana B. Cohen, co-author of the study, averred: "COVID-19 is complex and involves not only its toxic effect on cells but also on a complex set of systemic host responses. Therefore, cellular effects of drugs observed in a petri dish system may fail to translate to beneficial effects in people with COVID-19 as a result of a wide range of potential phenomena in whole organisms. Our trial reinforces the importance of not equating laboratory efficacy with clinical efficacy in the setting of COVID-19."

















