The scientific community has tried its best to end COVID-19's march. However, the SARS-CoV-2 virus has been evolving at a pace that is making it nearly impossible to keep up with. Several variants of concern (VOCs) have developed mutations that provide them with the ability to evade immunity acquired through vaccination as well as infection. Now, scientists have reported the development of a protein that can neutralize the virus and its variants.
In a multi-institutional study, researchers have described a new fusion protein utilizing the ACE2 (Angiotensin-converting enzyme 2) enzyme that the SARS-CoV-2 virus uses to infect cells. The protein—FYB207—combines ACE2 and fragments of natural antibodies known as human immunoglobulin G (IgG). In the in vitro study, FYB207 was found to completely block several variants of the virus (including VOCs) and prevent infection.
"These variants of concern are inhibited at picomolar concentrations proving that ACE2-IgG4 maintains – in contrast to therapeutic antibodies - its full antiviral potential. Thus, ACE2-IgG4-Fc fusion proteins are promising candidate anti-antivirals to combat the current and future pandemics," wrote the authors in the study that was published in the journal Science Direct.
Evading Immune Responses
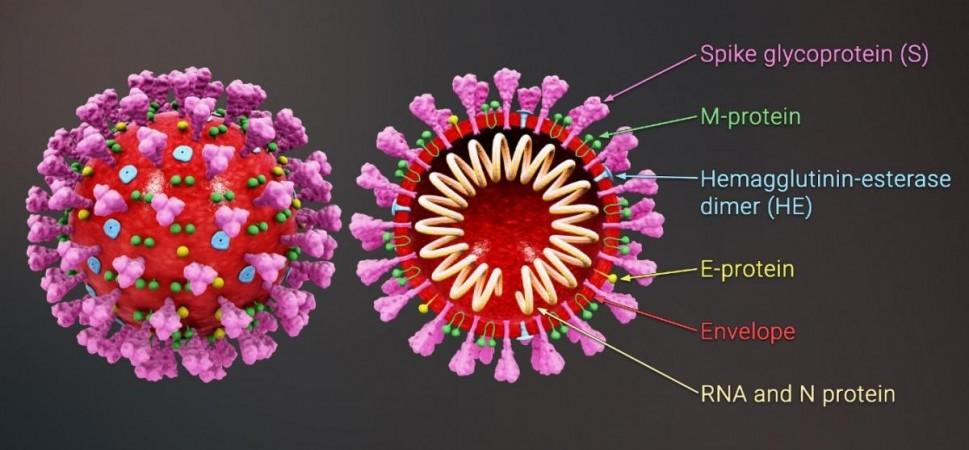
It is common knowledge that the SARS-CoV-2 virus uses its 'spike'—a hook-like glycoprotein found on its surface—to invade cells. It does so by binding or 'docking' with the ACE2 protein that is found on the cell membranes of cells in the lungs, intestines, arteries, kidneys, and heart. The SARS-CoV-1 virus, which causes SARS (severe acute respiratory syndrome), also employs the same mechanism.
Currently, vaccination is the primary weapon in the fight against COVID-19. However, newer variants of concern (VOCs) such as Delta and Omicron have been found to exhibit immunoevasive capabilities in vaccinated individuals as well as those who have recovered from the disease.
As far as treating the infection goes, recombinant antibodies—along with repurposed antivirals, and symptomatic management—have already been leveraged to tackle the respiratory infection in patients. However, much like its adaptations against natural antibodies, the SARS-CoV-2 has learnt to escape attacks by therapeutic antibodies also.
"Both vaccines and antibody medications have the same problem, that the virus manages to evade them by just a little bit more with each successful mutation. This results in what are called immune escape variants," explained Dr. Ulrike Protzer, co-lead author of the study, in a statement.
Neutralizing Multiple Variants
Through the current research, the team chose to focus on a different strategy: Why not target the ACE2 receptor itself? The study commenced with the evaluation of several combinations of ACE2 and fragment crystallizable (Fc) portions of human immunoglobulin G (IgG) antibodies as fusion proteins.
Fc is the tail section of an antibody that interacts with receptors known as 'FC receptors' found on the surface of cells. This approach of fusing proteins was made use of as ACE2—on its own—can be broken down easily by other enzymes in the body. Following this, the authors zeroed in on the IgG4 protein fragment, which is considered a dependable fusion partner.

Next, they introduced tiny mutations to boost the stability of the newly engineered ACE2-IgG4-Fc fusion protein. The action of the protein, dubbed FYB207 by the researchers, was tested against the original virus, as well as VOCs—Alpha, Beta, and Delta. Cell culture tests revealed that the new fusion proteins were able to neutralize all the examined variants of the SARS-CoV-2 virus and prevent infection. It did so by blocking the lethal spike proteins of the virus and its variants.
Highlighting the potential of the protein in tackling future variants, Dr. Buchner stated: "As the virus needs optimum docking on the ACE2 protein in order to survive, the virus cannot evade a medication which is based on exactly this protein. The fusion protein will therefore also be reliably effective against future mutations."
Uses Beyond Coronaviruses
While FYB207 could potentially be used to target other coronaviruses that utilize ACE2 as a docking point, it may also help in offering protection against threats of organ failure in the lungs, heart, and kidneys. This is because the receptor is known for its natural enzyme activity within the cardiovascular system.
Noting its ease of production, Dr. Carsten Brockmeyer, co-author of the study, said, "The fusion protein can be easily created biotechnologically." He also expressed hope of beginning clinical studies in the first half of 2022.
Two variants of FYB207 are currently being investigated in pre-clinical studies. Also, the researchers are set to begin testing the efficacy of the fusion protein against the new Omicron variant soon. Dr. Protzer emphasized that humanity will be challenged by the SARS-CoV-2 virus and its variants in the future.
"Even if vaccination is a highly reliable way to prevent severe symptoms in the course of the illness, the significantly more contagious Delta and Omicron variants have shown that both recovered and vaccinated patients can be re-infected. With regard to future, possibly even more contagious variants, we need a broadly efficacious active ingredient against the virus in addition to vaccination," concluded Dr. Protzer








