The COVID-19 pandemic can literally end if the SARS-CoV-2 virus can be prevented from binding with a particular receptor on human cells. However, the coronavirus' and its new variants' strength lies in their ability to do this deftly. Now, a new study states that targeting a protein molecule known as GRP78 can help inhibit the binding process, thereby, serving as a new potential target for treatment.
According to researchers from the Keck School of Medicine of USC, targeting GRP78—which is known for its role in enabling viruses to bind with human cells— can serve as a treatment target to contain the spread and severity of COVID-19 infections. The study provides the first experimental evidence supporting computer model predictions that have demonstrated GRP78's role in binding with cells, particularly in more infectious variants of SARS-CoV-2.
"Our study reveals that therapy targeting GRP78 could be more effective at protecting and treating people who contract COVID-19 than vaccines alone, particularly when it comes to people who can't get the vaccine and variants that could bypass vaccine protection but still depend on GRP78 for entry and production," said Dr. Amy S. Lee, senior author of the study, in a statement.
Cracking Under Stress

GRP78 is a molecular chaperone. A molecular chaperone is a protein that helps in the correct folding of protein subunits within a cell, particularly when the cell is under stress. However, some chaperones can be hijacked by viruses that use them to target and infect cells, where they ultimately reproduce and spread. GRP78 had been found to be susceptible to this misuse by other deadly viruses such as Zika and Ebola.
In the last 18 months, it has been established without a doubt that the SARS-CoV-2 uses its 'spike' protein (a hook-like structure on its surface) to bind with enzymes called ACE2 receptors (Angiotensin-converting enzyme 2) found on the surface of cells, to invade human cells. As a chaperone molecule, GRP78's function is to fold proteins in the protein production factory of cells—the endoplasmic reticulum (ER).
Under cellular stress, GRP78 is shipped to the surface of the cell, where they can aid in the binding of viruses with their specific target receptors. Upon entering the cells, the viruses hijack the ER protein folding machinery and replicate. With GRP78's known role in helping other viruses bind with cells, the authors investigated whether the molecular chaperone had a role in the invasion of cells by the novel coronavirus.
Doing More Than Its Job
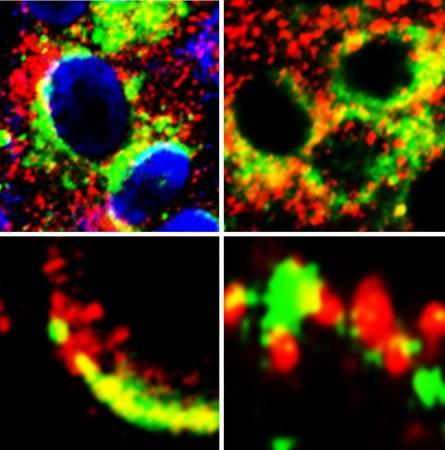
In order to examine the role of GRP78 in COVID-19 infection, the team treated lung epithelial cells with hMAb159, a humanized monoclonal antibody. hMAb159 is known for its action of removing GRP78 from the cell's surface without causing any harmful effects in mouse models. This treatment led to the removal of GRP78 and led to the reduction of ACE2 on the cell surface. This effectively caused the number of targets for the coronavirus to attach to diminish.
The researchers discovered that the GRP78 acts as a co-receptor and a stabilizing agent between SARS-CoV-2 and the ACE2 receptor. This enhanced the ACE2's recognition of viral spike protein and facilitating improved entry into host cells. In addition to this, the team learnt that GRP78 also binds to ACE2 and acts as its regulator—bringing the protein to the surface of the cell. This provides the SARS-CoV-2 more locations to bind with and invade cells.
How Does COVID-19 Hijack GRP78?
When under stress caused by the novel coronavirus infection, GRP78 is dispatched to the cell surface. Here, it promotes the binding process between the viral spike protein of SARS-CoV-2 and ACE2 receptors, thereby, facilitating enhanced entry into cells. After entering the cells and hijacking the ER mechanism like other viruses, the SARS-CoV-2 produces more viral proteins and replicates.
Interestingly, cellular stress is found to be higher in individuals with diseases such as cancer and diabetes. These conditions are known to increase susceptibility to COVID-19. Therefore, this may serve as an explanation for why pre-existing can increase the risk of the viral infection and the severity of its outcomes.
Potential Drug Target
GRP78 is required in limited amounts for healthy cells to survive. Nevertheless, disease-led stressed cells require a higher quantity of GRP78 to survive and replicate. According to the authors, interventions such as monoclonal antibodies and other agents can reduce the amount of GRP78 in the body, and may help in reducing the spread and severity of COVID-19 infection without any accompanying adverse effects.
The scientists also suggested that it can provide immunosuppressed individuals who fail to develop effective immune responses even after vaccination, with additional protection against COVID-19. "What is particularly exciting for this finding is that GRP78 could be a universal target in combination with existing therapies not just to combat COVID-19, but other deadly viruses that depend on GRP78 for their infectivity as well," noted Dr. Lee. The team intends to further explore the latest findings using animal studies next.

















