Though the COVID-19 pandemic is in its second year, effective treatment against the SARS-CoV-2 virus remains elusive. The therapeutic regimens to manage the disease are directed towards symptomatic treatment. However, offering a potential weapon to take the virus head-on, a new study reports the development of an experimental antiviral therapy that attacks the virus's genome directly.
Developed by an international team of researchers, the therapy uses a novel lipid nanoparticle (LNP) delivery system and also utilizes a gene-silencing RNA technique known as siRNA (small-interfering RNA). The antiviral therapeutic was found to improve survival rates in mice and effective viral clearance in the survivors.
"Treatment with virus-specific siRNA reduces viral load by 99.9 percent. These stealth nanoparticles can be delivered to a wide range of lung cells and silence viral genes," said Dr. Nigel McMillan, co-lead author of the study, in a statement.
RNA is the Key
The intentional outcomes of antivirals are the reduction of symptoms and quicker recovery. Currently, drugs such as Remdesivir are said to help benefit COVID-19. Remdesivir works by impeding the replication of the SARS-CoV-2. However, there are no existing drugs that can seek out the genome of the novel coronavirus and attack it directly.
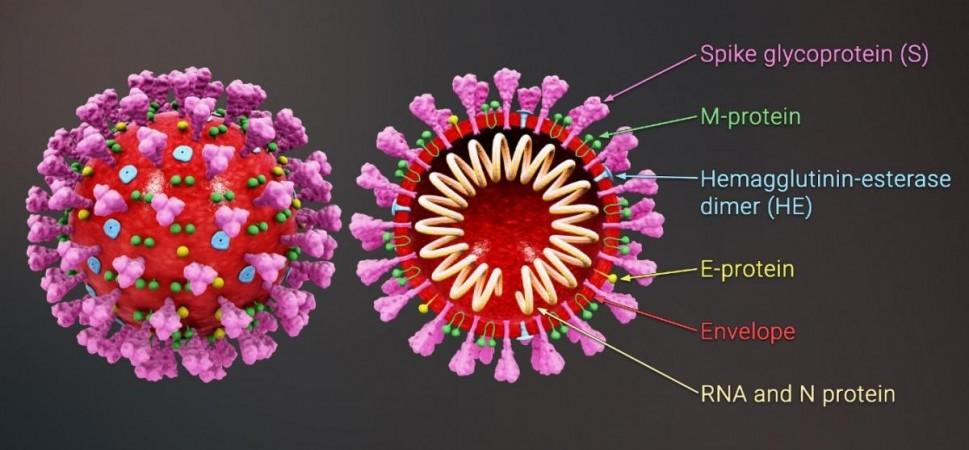
Coronaviruses such as the SARS-CoV-2 and SARS-CoV-1 are known as RNA viruses as RNA forms their genetic material. A coronavirus gains entry into a human cell using the 'spike'—a protein structure on its surface After invading the cell, the virus produces copies of itself using an enzyme called RNA-dependent RNA polymerase (RdRp).
Being RNA viruses, coronaviruses are vulnerable to RNA Interference (RNAi), which is the process of gene suppression in double-stranded RNA. siRNA are synthetic RNA molecules that are engineered to target a particular Messenger RNA (mRNA) for degradation. Therefore, the authors screened siRNAs that targeted specific regions of SARS-CoV-2 to block its expression and replication.
Attacking the Virus's Genome
In order to glean the potency of RNAi of SARS-CoV-2, the team developed several siRNAs that targeted highly-conserved regions of the virus, especially the RdRp. Among the numerous designed siRNAs, three candidates exhibited effective inhibition of the virus by over 90 percent. This efficacy was either alone, or in combination with each other.
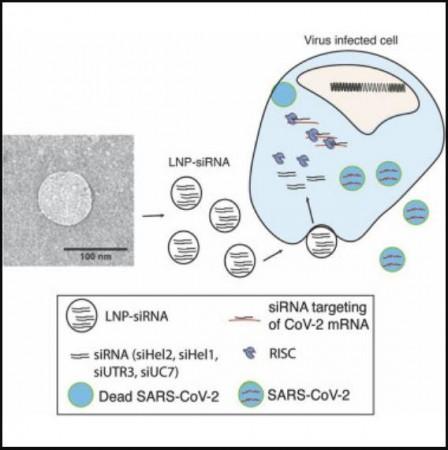
The authors designed LNP formulations that could serve as the delivery system of the chosen siRNAs. Two candidates emerged as ideal options for delivering the siRNA therapeutics to the lungs—the organ most affected by COVID-19. Next, the authors treated infected mice with the LNP-encapsulated siRNAs (or sLNP-siRNAs) which were administered through an injection.
Promisingly, powerful suppression of the virus was observed in the lungs of the mice. The sLNP-siRNAs were also found to provide the treated mice a pronounced survival advantage. "Treatment with the therapy in SARS-Cov-2 infected mice improved survival and loss of disease. Remarkably, in treated survivors, no virus could be detected in the lungs,'' stated Dr. McMillan.
Beyond Just COVID-19
While the new antiviral has exhibited high potency against SARS-CoV-2, the authors affirm that it can be used against its variant and other viruses as well. "This treatment is designed to work on all betacoronaviruses such as the original SARS virus (SARS-CoV-1) as well as SARS-CoV-2 and any new variants that may arise in the future because it targets ultra-conserved regions in the virus' genome," asserted Dr. Kevin Morris, co-lead author of the study.
According to the authors, the production of the nanoparticles (sLNP-siRNA particles) is not only scalable but also comparatively cost-effective to manufacture in bulk. "We have also shown that these nanoparticles are stable at 4°C for 12 months and at room temperature for greater than one month, meaning this agent could be used in low-resource settings to treat infected patients," added Dr. McMillan.
Therefore, the results of the study demonstrate that siRNA-nanoparticle formulations can potentially be developed to treat COVID-19 patients. Most importantly, they could serve as a crucial tool against other coronavirus infections that may arise in the future.















