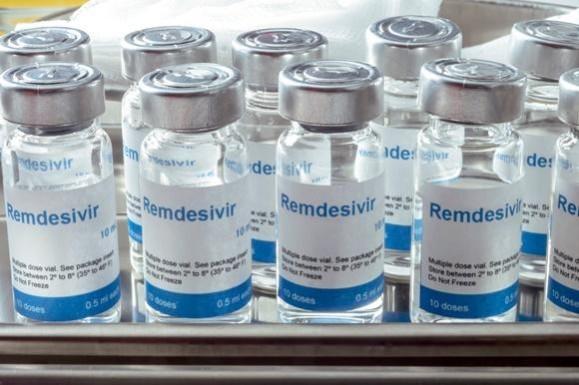
As cases of coronavirus are rising rapidly in the country, two crucial life-saving drugs, remdesivir and tocilizumab are in short supply in hospitals and people are being forced to buy it at an exorbitant rate on the black market.
Remdesivir, the only drug approved for emergency use for patients admitted in hospitals with severe complications due to COVID-19, has become the prime medication for patients.
Likewise, tocilizumab, sold as Actemra, is in short supply at hospitals. The drug has also shown positive results in critically ill patients around the world.
Not in hospital but remdesivir available on black market
According to media reports and several testimonials by people, the drug remdesivir and tocilizumab are either not available in hospitals or available at an exorbitant price on the black market.
In one investigation by BBC, some patients' relatives have been asked by the doctors to procure remdesivir themselves as there is no drug in hospitals. Desperate, patients' relatives are forced to buy remdesivir on the black market at exorbitant prices.
Click here for coronavirus related stories
Having gone through the plight, Abhinav Sharma, whose uncle was admitted to the hospital in Delhi and tested positive for COVID-19, said, he desperately tried to get the remdesivir as the condition of his uncle started to deteriorate.
But even after several attempts, Mr Sharma says he couldn't procure the drug as it wasn't available anywhere.
"I had tears in my eyes. My uncle was fighting for his life and I was struggling to arrange the medicine that could possibly save him," he told BBC.
"After dozens of calls, I paid seven times the price to get the medicine. I was willing to pay any price really, but my heart goes out to people who can't afford it," he said.
Many citizens across the country facing the shortage of remdesivir
Mr's Sharma's not the only case. There are many families in Delhi and several other parts of the country who have complained about the unavailability of remdesivir in hospitals and that they have been forced to buy from the black market.
The rise in the demand for remdesivir is driven by the fact that it has shown to reduce the treatment duration from 15 days to 11 days in clinical trials around the world.
Remdesivir approved only for emergency use in hospitals
Though it has only been approved for emergency use for critical patients admitted in hospitals, and not be given to all COVID-19 patients, in absence of any proven drug, doctors are increasingly prescribing it to patients.
This in addition to the shortage of the drug, has raised the price of remdesivir exponentially.
While the official price of a single vial of the antiviral drug remdesivir is around Rs 5,400, it is being sold in the range of 15 to 60 thousand per vial in the black market. And, as for complete course, a patient needs at least 6 vials for the treatment of COVID-19, the total cost for 6 vials could go much higher depending on the price it is bought in the black market.
It is not surprising that in a desperate moment, family members are forced to pay the high price, in some cases spending their life's saving in an effort to save the life of their loved ones.
Only one Indian company is making remdesivir as of now
It can be noted that remdesivir has originally been developed by Gilead Sciences, a US pharmaceutical company, for treating Ebola patients. But it has also proven to be effective on COVID-19 patients in clinical trials though there have been other side effects of it.
For the Indian market, Gilead Sciences has collaborated with four local pharmaceutical companies to manufacture the generic version of the drug remdesivir.
But of four-Cipla, Hetero Drugs, Jubilant Life and Mylon- only Hetero has so far produced 20,000 of doses of remdesivir and distributed it amongst five states.
How remdesivir reached on black market
As part of their investigation, when BBC asked Hetero how drugs have reached in black market when there's a shortage of the drug in the hospital, Hetero representative said it wasn't sure how the "leakage" was happening.
"We have not given the medicine to our distributors. As per the guidelines, we have directly supplied the vials to hospitals," Hetero's vice-president of sales Sandeep Shahstri said.
He added that the company is working hard to provide the drug to meet the demand and that "such black marketing was really demoralising".
"We understand the pain of the families. They shouldn't be told to go and hunt for the drug. We are confident of increasing our production in the next few days and the situation should get better," he told the BBC.
Chemists association too denied the knowledge about the "leakage".
General secretary of All India Chemists and Druggists Association, Rajiv Singhal, also denies that any chemist or shop owner is involved in it.
"I am sure that none of our members is involved in such practices. It's a national health emergency and I want to give a clear message that strict action will be taken against if anyone is found to be selling life-saving medicines illegally," he said.
Drug Controller of India takes action
LocalCircles, a community engagement and social media platform, who keep a watch on social issues raised the issue with the Ministry of Health.
The LocalCircles in its letter to the Ministry of Health said that it received many complaints from citizens across the country about the alleged black marketing of Remdesivir.
"The MRP of Remdesivir marketed by Hetero Healthcare Limited is Rs 5,400 but consumers have reported it being sold at a price of anywhere between Rs 15,000 to 60,000," FE reported the LocalCircles saying.
Taking cognisance of the issue, the Drug Controller General of India asked drug controllers in states and Union Territories to keep a strict vigil to prevent black marketing of the anti-viral Remdesivir injection.
Last week, the US had procured almost 90 per cent of the world's supply of remdesivir from Gilead Sciences for patients in US hospitals.










![BJP fields Tashi Gyalson for Ladakh; drops sitting MP [details]](https://data1.ibtimes.co.in/en/full/797185/bjp-fields-tashi-gyalson-ladakh-drops-sitting-mp-details.jpg?w=220&h=138)



