The central government has adopted a policy of allowing foreign vaccines immediately to increase the number of Corona vaccines in the country. The Centre said today that the process of approving emergency use in India of those foreign Covid vaccines that are being used abroad will be expedited. It is to be noted that the Drug Controller General of India (DGCI) has approved the emergency use of Sputnik V, a COVID-19 vaccine developed in Russia. The Expert Committee had on Monday recommended Sputnik V to approve emergency use in India.
The entities the government has named in its order are associated with the US, Europe, Britain, Japan, and WHO. Those approved of the vaccine include the US Food and Drug Administration, the European Medicine Agency, UKMHRA, PMDA Japan, and the World Health Organization.
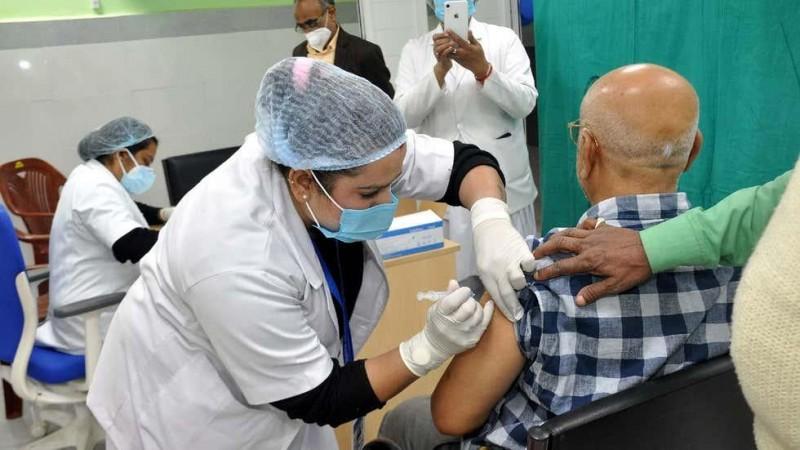
In an official statement, the Health Ministry said, "The National Expert Group on Vaccine Administration for COVID-19, after comprehensive deliberation, recommended that vaccines, which have been developed & are being manufactured in foreign countries and which have been granted emergency approval for restricted use by USFDA, EMA, UK MHRA, PMDA Japan or which are listed in WHO(Emergency Use Listing) may be granted emergency use approval in India, mandating the requirement of post-approval parallel bridging clinical trial in place of conduct of local clinical trial as per the provisions prescribed under Second Schedule of the New Drugs & Clinical Trials Rules 2019."
The vaccine that the government will approve will be tested on 100 patients for the next 7 days. After that, the country will be included in the vaccination program. The government claims that this decision will help in importing the vaccine in India and accelerating the vaccination program.
Vaccine roll out to be fast-tracked
According to experts, the vaccine dose becomes available to the common people within a week and a half after the approval of emergency use. Further, if there is no major obstacle, then the vaccine dose can be given within 10 days of approval. Currently in the country, in addition to Covaxin, developed by the joint efforts of Bharath Biotech and Indian Council of Medical Research (ICMR), doses of Covishield developed by Oxford University and AstraZeneca in addition to Covaxin are being used to vaccinate the population.








