Malaria is one of the worst mosquito-borne diseases in the world. It is responsible for over 400,000 deaths worldwide every year, with children being most vulnerable to the disease. However, the malaria parasite can survive high fevers that are launched against it as a response by the body. The mechanism behind it remains unknown. Now, scientists have discovered a gene that bestows the parasite with the ability to defend itself against adverse conditions within the host body, including high fever temperatures.
A study led by the Barcelona Institute for Global Health (ISGlobal), has reported that a newly-discovered gene, PfAP2-HS, regulates the protective heat-shock responses of Plasmodium falciparum, one of the most lethal malaria-causing pathogens. The research was published in the journal Nature Microbiology.
"This is the first transcription factor described in Plasmodium capable of regulating responses to adverse host conditions, including fever. PfAP2-HS acts as 'an orchestra director', coordinating the other proteins involved in the response," said Dr. Alfred Cortés, corresponding author of the study, in a statement.
A Global Killer

Malaria is caused in human beings by five parasitic protozoa from the Plasmodium family. They are transmitted through the bites of infected female Anopheles mosquitoes that are primary vectors. Two of these protozoans—Plasmodium falciparum and Plasmodium vivax—pose the biggest threats. Malaria causes several complications including damage to organs such as the kidneys and the liver, and can also result in the rupturing of the spleen.
According to the World Malaria Report released by the WHO in 2020, there were 229 million cases of malaria in 2019. The estimated number of deaths due to malaria was 409,000 in 2019. Children below the age of five accounted for 67 percent (274,000) of all malaria deaths.
Countering Pathogens with Fever
Infections caused by P. falciparum are marked by periodic fevers. This occurs every time a cycle of asexual reproduction of P. falciparum ends and a new batch of parasites are released into the bloodstream. These elevations in body temperatures arise as fever is an integral component of the body's response to invading pathogens; impeding the balance of their cellular proteins and enabling reduction of the parasite load or burden.
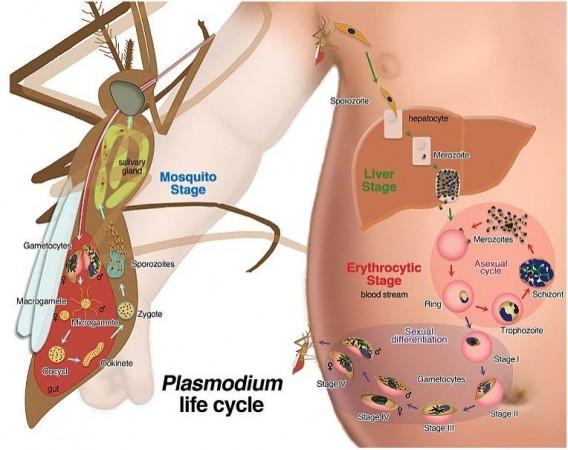
As a result, several pathogens have developed defences against increased temperatures—the expression of heat-shock proteins (HSP), which act as chaperones that assist in the process of survival. "In most of the eukaryotic organisms, from yeasts to mammals, the expression of these proteins depends on a highly conserved transcription factor called HSF1. However, malaria parasites – which are also eukaryotes - lack the HSF1 gene, although we know that they can survive at febrile temperatures," explained Dr. Cortés.
Surviving High Temperatures
Through the study, the authors sought to understand the manner in which the parasite moderates its response to elevated temperatures (also known as 'heat shock') in spite of HSF1 being absent. It was noted that a P. falciparum cell line—which was cultivated in the laboratory—lost its ability to survive exposure at a temperature of 41.5 C. This was on account of a mutation in a gene that the team named PfAP2-HS.
The researchers also demonstrated that PfAP2-HS functions as a transcription factor that triggers the activation of heat shock proteins hsp90 and hsp70-1. It did so by binding with the respective 'promoters'—the 'on-off' switch of a gene—of the two genes. Additionally, the scientists also showed that parasites that were engineered to lack the PfAP2-HS gene exhibited a lower survival when exposed to higher temperatures, along with decreased growth at a 'normal' temperature of 37 C.
Dr. Elisabet Tintó-Font, first author of the study stated, "This means that, in addition to its role in the protective heat-shock response, PfAP2-HS is also important for maintaining protein stability in the parasite at basal temperatures."
Susceptibility to Drugs
Another important finding of the study was that the absence of PfAP2-HS in P. falciparum increased its susceptibility to drugs due to changes in protein stability. When tested against artemisinin (a frontline antimalarial drug) and epoxomicin (a proteasome inhibitor), the P. falciparum lacking PfAP2-HS showed high vulnerability.
The authors also found homologues of PfAP2-HS—a gene similar to genes in other species—in all the analyzed species of Plasmodium, including those that infect mice and do not result in fever. "This suggests that, at least in those species, the response orchestrated by AP2-HS could protect against other adverse conditions in the host," highlighted Dr. Cortés.










!['Lip lock, pressure, pyaar': Vidya Balan- Pratik Gandhi shine in non-judgmental infidelity romcom Do Aur Do Pyaar [ Review]](https://data1.ibtimes.co.in/en/full/797104/lip-lock-pressure-pyaar-vidya-balan-pratik-gandhi-shine-non-judgmental-infidelity-romcom.jpg?w=220&h=138)






